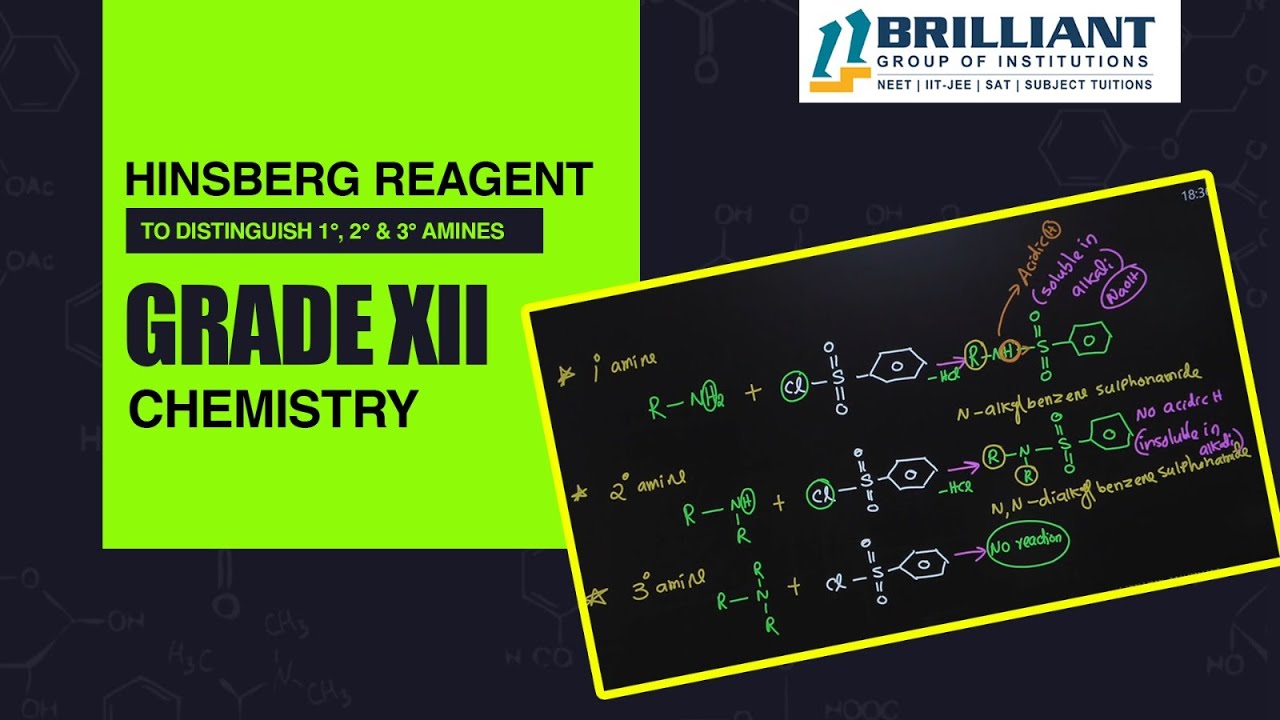
In this lecture, we discuss how to distinguish primary, secondary, and tertiary amines using the Hinsberg reagent.The video was created in accordance with the latest syllabus for CBSE Class 12 Chemistry – Hinsberg Reagent published by Brilliant Qatar.
The Hinsberg reaction is a test for the detection of primary, secondary and tertiary amines. In this test, the amine is shaken well with Hinsberg reagent in the presence of aqueous alkali (either KOH or NaOH). A reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride is added to a substrate. A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent(benzene sulfonyl chloride) and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt. In this way the reaction can distinguish between the three types of amines.